SARS-CoV-2 (COVID-19) IgG ELISA Kit
Original price was: $1,140.00.$1,089.00Current price is: $1,089.00.
SARS-CoV-2 (COVID-19) IgG is intended for the qualitative determination of IgG class antibodies against SARS-CoV-2 in human serum or plasma (citrate, heparin) to support the diagnosis of COVID-19 disease and constitutes a supplement to direct pathogen detection. In addition, serology can be used to collect epidemiological information on the prevalence of SARS-CoV-2.
Precision
| Sample | n | Mean | SD | CV% |
|---|---|---|---|---|
| No. 1 | 24 | 4.06% | ||
| No. 2 | 24 | 4.28% | ||
| No. 3 | 24 | 8.71% |
| Sample | n | Mean | SD | CV% |
|---|---|---|---|---|
| No. 1 | 12 | 7.37% | ||
| No. 2 | 12 | 4.11% | ||
| No. 3 | 12 | 8.65% |
-
Sample type
Serum, Hep Plasma, Cit plasma -
Assay duration
Multiple steps standard assay -
Product overview
SARS-CoV-2 (COVID-19) IgG is intended for the qualitative determination of IgG class antibodies against SARS-CoV-2 in human serum or plasma (citrate, heparin) to support the diagnosis of COVID-19 disease and constitutes a supplement to direct pathogen detection. In addition, serology can be used to collect epidemiological information on the prevalence of SARS-CoV-2.
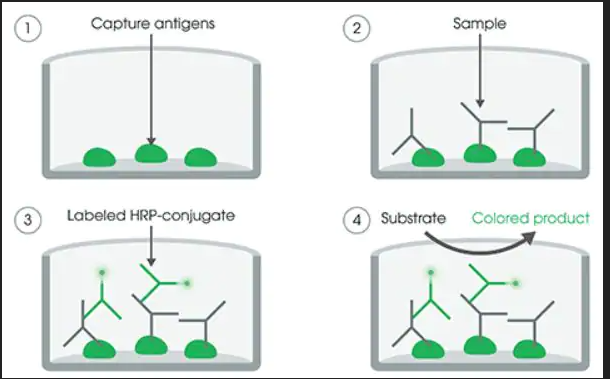
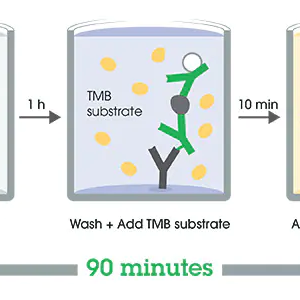
Microtiter plates are coated with specific antigens to bind corresponding antibodies of the sample. After washing the wells to remove all unbound sample material horseradish peroxidase (HRP) labelled conjugate is added. This conjugate binds to the captured antibodies. In a second washing step, unbound conjugate is removed. The immune complex formed by the bound conjugate is visualized by adding Tetramethylbenzidine (TMB) substrate which gives a blue reaction product. The intensity of this product is proportional to the amount of specific antibodies in the sample. Sulfuric acid is added to stop the reaction. This produces a yellow endpoint color. Absorbance at 450/620 nm is read using an ELISA Microtiter plate reader.
-
Notes
Antibody Isotypes and the State of Infection:
Serology Significance IgM Characteristic of the primary antibody response. High IgM titer: → suggests a current or very recent infection.
IgG Follows IgM production. Characteristic of the secondary antibody response.
May persist for several years.
High IgG titer with low IgM titer: → may indicate past infection.
IgA Produced in mucosal linings throughout the body (⇒ protective barrier). Usually produced early in the course of the infection.
Diagnostic Specificity:
The diagnostic specificity is defined as the probability of the assay of scoring negative in the absence of the specific analyte. SARS-CoV-2 infections emerged in December 2019 in Wuhan, China. The expected prevalence values for German and US blood donor panels from before December 2019, therefore, amount to 0 %. The determined positive results correspond to a specificity of 99.24 % (95 %-confidence interval: 95.82 % – 99.98 %).
Sample panel No. patients (n) Positive Equivocal Negative Specificity 95% CI Blood donors Germany 83 0 1 82 100% Blood donors US 50 1 0 49 98% Total 133 1 1 131 99.24% 95.82%-99.98% Diagnostic Sensitivity:
The diagnostic sensitivity is defined as the probability of the assay scoring positive in the presence of the specific analyte. 42 samples from 25 patients tested positive for SARS-CoV-2 RNA by RT-PCR.
Days post symptom onset No. samples (n) Positive Equivocal Negative Sensitivity (Eqv excluded) 0-5 13 1 0 12 7.69% 6-8 10 4 0 6 40% 9-11 10 4 0 6 40% greater than 12 9 9 0 0 100% Interferences:
Three clinical samples exhibiting differing reactivities were tested for interference with each substance listed in the Table below: a positive, a negative, and an equivocal sample. All samples exhibited a change of signal less than 15 % when tested with each potential interferant.
Interferent Conc. tested Albumin 60 mg/mL Bilirubin conjugated 0.4 mg/mL Bilirubin unconugated 0.4 mg/mL Cholesterol 4 mg/mL Hemoglobin 10 mg/mL Triglycerides 15 mg/mL